Introduction
Preimplantation genetic testing (PGT) involves analysis of the DNA from oocytes (polar bodies) or in vitro fertilized embryos (cleavage stage or blastocyst) for determining genetic abnormalities or for HLA typing (Zegers-Hochschild et al., 2017). This includes three modalities—PGT for aneuploidy (PGT-A), PGT for monogenic/single gene defects (PGT-M) and PGT for chromosomal structural rearrangements (PGT-SR).
Chromosomal mosaicism (defined as a state in which there is more than one karyotypically distinct cell population arising from a single embryo (Zegers-Hochschild et al., 2017)) is an inherent biological phenomenon in human preimplantation embryos. Further details about the nature of the aneuploidy implicated in mosaicism and the mechanisms and incidence of chromosomal mosaicism have been addressed in recent reviews (Viotti, 2020; Levy et al., 2021).
Embryos with a mosaic result have been observed for a long time, for example when mosaicism involved the chromosome linked with a monogenic disease and single cell multiplex PCR showed one or three parental haplotypes. Following the implementation of high-resolution genome-wide methods, usually based on next-generation sequencing (NGS) of trophectoderm (TE) biopsies, the detection of intermediate copy number on chromosomal analysis (indicating chromosomal mosaicism among the biopsied cells) has become more frequent. As a result, data interpretation has become more challenging and embryo transfer policies more complicated.
In the context of PGT-A, the most relevant type of mosaicism is the mix of euploid and aneuploid cells (sometimes referred to as diploid–aneuploid mosaics). Embryos with chromosomally mosaic results after TE biopsy will be referred to as ‘mosaic’ in the remainder of this article. Embryos with ‘segmental gain/loss’ showing intermediate test results indicating aneuploid/diploid mosaicism are referred to as ‘segmental imbalances’ in the remainder of this article.
After the first report showing that the transfer of embryos with a chromosomal mosaic result on PGT-A can yield healthy babies (Greco et al., 2015), a growing series of studies has been published on this topic (compiled in Viotti, 2019), with the largest dataset of 1000 embryos described in Viotti et al. (2021b). These data suggested that the transfer of embryos with putative mosaic PGT-A results yielded lower implantation rates and higher miscarriage rates when compared with euploid embryo transfer.
There are several outstanding issues: the analytical validity of determining the presence of mosaicism is suboptimal, as the same intermediate copy number results can occur for biological and technical reasons that are unrelated to mosaicism (i.e. technical noise or ploidy abnormality in embryos with extra/missing chromosomes); the developmental potential of mosaic embryos as well as the clinical significance of mosaicism detected at the preimplantation stage of development remains unclear; the presence of mosaicism in a TE biopsy may not reflect the chromosomal constitution of the whole embryo; and information on the risks associated with specific types of mosaicism (e.g. affecting specific whole chromosomes, different numbers of chromosomes or segmental imbalances) is still insufficient. These issues make it difficult to guide clinicians and patients on the management of embryos scored as ‘mosaic’ or come to a uniform embryo ranking system.
So far, the PGD International Society (PGDIS) and Controversies in Preconception, Preimplantation and Prenatal Genetic Diagnosis (CoGEN) have issued position statements on the transfer of mosaic embryos (COGEN, Cram et al., 2019; Gleicher et al., 2020; Leigh et al., 2022). In parallel, the Practice Committee and Genetic Counseling Professional Group (GCPG) of the American Society for Reproductive Medicine has published guidance on how to counsel patients on the issue of embryo chromosomal mosaicism (Practice Committee and Genetic Counseling Professional Group (GCPG) of the ASRM, 2020).
This article aims to provide good practice recommendations on how to manage the detection of chromosomal mosaicism in clinical practice and, more specifically, provide guidance on the essential elements that should constitute the consent forms and the genetic report, and that should be covered in genetic counselling. The recommendations are supported by published data and the outcomes of a survey on practices in PGT laboratories and ART clinics with regards to detection and management of chromosomal mosaicism in embryos. Rather than providing instant standardized advice, the recommendations should help PGT centres in developing their own policy towards the transfer and cryopreservation of ‘mosaic’ embryos. These recommendations should be applied in consideration of previously published recommendations for organization of PGT (ESHRE PGT Consortium Steering Committee et al., 2020), polar body and embryo biopsy for PGT (ESHRE PGT Consortium and SIG-Embryology Biopsy Working Group et al., 2020), detection of structural and numerical chromosomal aberrations (ESHRE PGT-SR/PGT-A Working Group et al., 2020) and detection of monogenic disorders (ESHRE PGT-M Working Group et al., 2020). In addition to the recommendations, we have identified missing information and scientific questions, which should guide further research in PGT and chromosomal mosaicism.
Methodology
The current good practice recommendations have been developed according to the manual for development of ESHRE good practice recommendations (Vermeulen et al., 2019).
Data on current practice with regards to the detection and management of chromosomal mosaicism discovered during any PGT practice offering comprehensive screening (PGT-A, PGT-M + PGT-A and PGT-SR + PGT-A) were collected through a web-based questionnaire. The questionnaire, with mostly multiple-choice answers, had a separate ART (biopsy methods) and PGT (genetic testing methods) section and common sections on reporting embryo transfer policy and follow-up of transfers, pregnancies and children born (Supplementary Data SI). Identifying parameters (name of the unit, country, city, street and email) were included to identify and remove duplicate replies. Replies from members of special interest groups (SIG) (the ESHRE SIG Reproductive Genetics and SIG Embryology) were collected between 20 February and 9 April 2020 (7 weeks) as well as from PGT Consortium members.
Data on mosaicism and PGT published up to May 2022 were collected from the literature in a PubMed/MEDLINE search. Search terms included chromosomal mosaicism, mosaic embryo, mosaicism and PGT. Animal studies were excluded as well as papers published before 2010 and those not published in English. References retrieved from the literature review were complemented with further key references identified by the working group (WG) members.
The recommendations for clinical practice were formulated based on the expert opinion of the WG while taking into consideration the published data and results of the survey.
The final draft was published on the ESHRE website between 15 February and 16 March 2022 for stakeholder review. Eighty-four comments were received and incorporated where relevant. The review report is available on www.eshre.eu/guidelines.
Results
Current practice with regards to detection and management of chromosomal mosaicism
Three hundred and thirty-four replies were received. After exclusion of replies with insufficient identifying parameters (n = 22), duplicate replies (n = 26), blank submissions (n = 16) and replies that did not correspond to a centre offering PGT (either in-house or outsourced) (n = 31), the final dataset included 239 replies representing 239 centres (Supplementary Data SII). In considering the survey results, readers should be mindful that the replies were collected in 2020 and may be different following recently published data relevant to mosaicism.
Characteristics of the centres
Of the 239 centres participating in the survey, 53.6% were located in Europe, 24.3% in Asia and 8.7% and 10.0% in North and South America, respectively. Only a small number of participants were located in Africa and Oceania (1.25% and 2.1%, respectively). Within Europe, the largest number of participating centres was from Spain (n = 30) (Fig. 1A). Overall, 73.2% of the centres were private centres (Fig. 1B). Of the centres, 60.3% were ART centres outsourcing PGT, 32.2% combined ART and PGT activities and the remaining 7.5% were independent PGT centres not linked to a specific ART centre (i.e. performing PGT for several ART centres) (Fig. 1C). Worldwide, 84.1% (201/239) of participating ART/PGT centres are accredited/certified according to international and/or national standards (Fig. 1D).
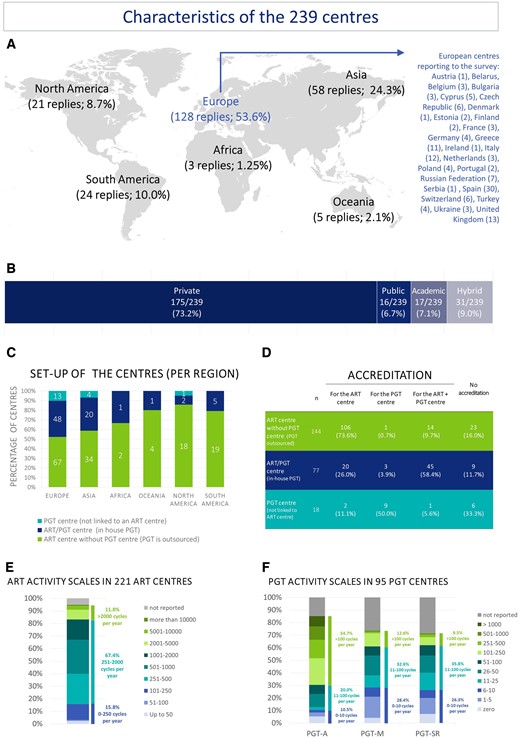
Characteristics of the 239 ART/PGT centres included in the survey. (A) The location, (B) type and (C) set-up of the centres are shown, together with (D) whether they are accredited, and the activity scales for ART (E) and PGT (F). PGT, preimplantation genetic testing; PGT-A, PGT for aneuploidy; PGT-M, PGT for monogenic/single gene defects; PGT-SR, PGT for chromosomal structural rearrangements.
The activity scale of the number of ART cycles varied from low (<50 cycles/year) to very high (more than 10 000 cycles/year) (median 700.0). About half of the centres (50.7%) carried out between 250 and 1000 ART cycles/year. Based on data for 194 centres (those reporting number of cycles for ART and at least PGT-A, PGT-M or PGT-SR), the percentage of ART cycles with any genetic testing is 24.2% ± 1.71 (mean ± SEM). This calculation assumes that blank fields are 0, the highest value was considered when a range was reported, and combined genetic testing was not taken into consideration. Half of the centres (50.5%) offering PGT-A perform 51–250 PGT-A cycles/year. Of centres offering PGT-M, the majority (61.0%) perform up to 100 cycles per year. For PGT-SR, 61.9% of centres perform 1–100 cycles/year (Fig. 1E and F). About half of the centres (47.7%) offer testing for all indication groups (PGT-A, PGT-M/PGT-A, PGT-SR/PGT-A); 14.2% of centres apply only PGT-A while 10% offer no PGT-A at all (Table I).
Table I
Overview of the indications for which PGT was performed with respect to the centre set up (ART centre, PGT centre or ART/PGT centre).
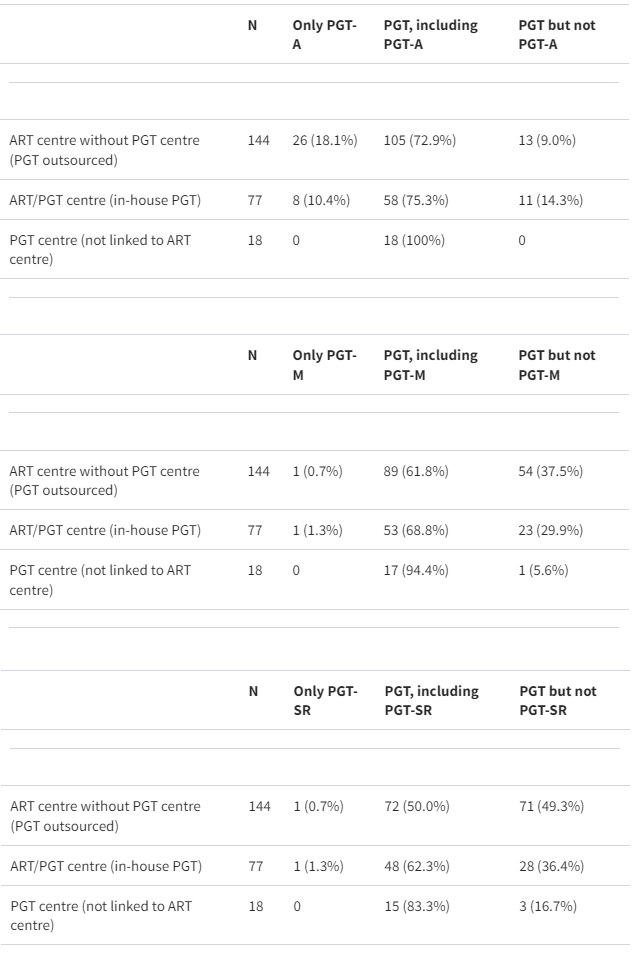
PGT, preimplantation genetic testing; PGT-A, PGT for aneuploidy; PGT-M, PGT for monogenic/single gene defects; PGT-SR, PGT for chromosomal structural rearrangements.
( Open in new tab )
Biopsy and PGT
For all indication groups, biopsy was most frequently applied at the blastocyst stage (on average 84.2%), followed by cleavage or blastocyst stage biopsy (on average 10.0%), based on replies from 212 centres. The biopsy stage hardly varied in relation to the PGT indication (Supplementary Data SIII). These figures are similar to the ones reported in the ESHRE PGT Consortium data collection papers and have stabilized at this level since 2017 (Coonen et al., 2020).
Laser-assisted drilling was the most commonly used method for zona breaching (86.8%). Alternatives included combinations of laser drilling and mechanical opening of the zona pellucida (8.0%) and laser drilling with acidic Tyrode’s solution (2.8%). Other approaches were used in < 1% of centres.
The majority of blastocyst/TE biopsies are carried out on Day 5 and/or Day 6 of in vitro development. In 33.3% of centres, Day 7 was included as an additional option for blastocyst biopsy. In 56.7% of centres, the optimal cell number aimed for in blastocyst biopsy is 5–10, while 36.4% of centres aim for 3–5 cells. A minority of centres aims for < 3 cells (3.7%) or more than 10 cells (3.2%). The latter reported biopsy of 8–12 cells (n = 1), 10–15 cells (n = 1) or >15 cells (n = 4).
PGT technologies
About half of the centres use shallow sequencing (i.e. very low depth whole-genome sequencing) as a single test strategy to provide information on PGT-SR and/or aneuploidy. For PGT-M, 35.2% of respondents indicate they employ a combination of different techniques, while for PGT-M/PGT-A, over 50% employ a combination of techniques (Supplementary Data SIV).
Of the centres performing in-house PGT (either PGT centres or ART/PGT centres), 88.1% (59/67) indicated that they had validated the technology for PGT, independently from manufacturer validation (Fig. 2A). In centres that outsource their genetic analysis, 45.5% (30/66) indicated that a validation of the technology was performed. Of those performing a validation of the technology (independent of the type of centre), 61.8% (55/89) included the calling of chromosomal mosaicism.
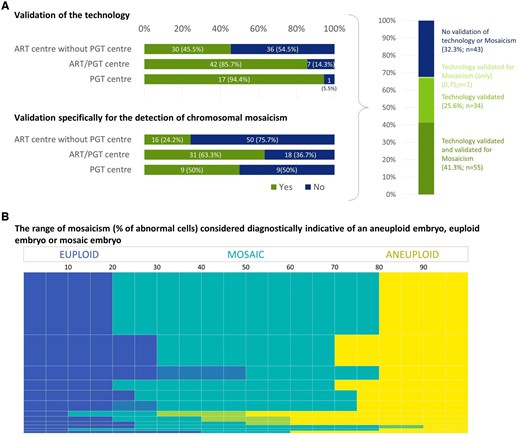
Figure 2.
Validation of the methods for detection of aneuploidy and mosaicism, and definition of the percentage of abnormal cells considered indicative of a mosaic embryo. (A) Combination of the replies to the question (yes/no) on whether the centres have validated the technology they are using and whether they have validated the technology specifically for mosaicism (yes/no) (data categorized per centre set-up and presented as numbers and percentages). (B) The reported percentage of abnormal cells indicative for an euploid (blue), mosaic (teal) and aneuploid result (yellow). The height of the bar reflects the number of centres stating the presented range. Merging of similar replies is indicated by blended colours indicating the range in which replies varied. Out of a total number of 114 replies, 92 are included in the figure. The remaining 22 replies were considered irrelevant or incorrect, likely a result of misunderstanding the question.
Ninety-two centres provided valid replies when asked about the range of mosaicism (percentage of abnormal cells) that they consider diagnostically indicative of an aneuploid embryo, euploid embryo or mosaic embryo. Of these centres, 39.1% (36/92) use a cut-off level of ≤20% abnormal cells to designate a euploid embryo and ≥80% abnormal cells for an aneuploid embryo; 19.6% (18/92) use cut-off levels of ≤30% and ≥70%, respectively (Fig. 2B). The remaining 38 centres use a variety of other cut-off levels. Interestingly, 58.7% (54/92) of the centres specifying a range had not validated their technology for detecting mosaicism. We have inferred the threshold for detecting mosaicism based on these definitions, i.e. a centre giving a cut-off level of 20% would report a sample with 20% of abnormal cells.
Reporting
About 40% of the centres (39.1%; 79/202 replies) include full information on aneuploidy results in their PGT reports (including whole chromosome aneuploidy, segmental imbalances (gain/loss) and intermediate copy number results) over all indication groups, while most of the others report aneuploidy (but not mosaicism and segmental imbalances). When segmental imbalances are reported, roughly half of the centres (53.9%, 82/152) specify the resolution of their technology. Of the centres that report mosaicism, 71.0% (71/100) specify the degree of mosaicism (Fig. 3A-C). Centres biopsying one or two cells did not consider reporting of mosaicism as being useful; when three or more cells are biopsied, 80% of centres report mosaicism.
The genetic report includes a recommendation or prioritization for embryo transfer in 60.9% of centres. For one-third of centres (67/202), there is a recommendation about which embryos are suitable for transfer and in 19.3% (39/202), there is a ranking of the embryos as well (Fig. 3D). Forty-nine percent of the centres (99/202) stated that they take mosaicism in consideration when making a recommendation for embryo transfer. With regards to specific criteria considered for prioritization of mosaic embryos, 93 replies were received of which 9.7% (9/93) emphasized the need for genetic counselling for prioritization, without providing any further details. The criteria mentioned in the remaining 84 replies involved level of mosaicism (i.e. the percentage aneuploid cells (Viotti, 2020)), the type of mosaicism (i.e. involving segmental, versus whole chromosome, versus complex abnormalities (Viotti, 2020)), the type of chromosomes involved (e.g. association with potential for uniparental disomy (UPD), severe intrauterine growth retardation or liveborn syndromes (Cram et al., 2019)) and the number of chromosomes involved, either as sole criterion or in combination. The most commonly provided answer mentioned a combination of level of mosaicism and type of chromosome involved (32.1%; 27/84), followed by level of mosaicism as a sole criterion (27.3%; 23/84), followed by type of chromosome as a sole criterion (11.9%; 10/84) and type of mosaicism as a sole criterion (3.6%; 3/84). A combination of level of mosaicism, type of chromosome and type of mosaicism was reported in 4.8% (4/84) of answers, a combination of level of mosaicism, type of chromosome and number of chromosomes in 5.9% (5/84), and a different combination of the above criteria in 7.1% (6/84) of answers. Finally, 7.1% (6/84) referred to the PGDIS recommendations.
Figure 3.
Information on chromosomal status included in the PGT report in different centres. (A) Overview of whether the centres report aneuploidies, mosaicism or both. Replies were collected per indication for which PGT is performed. The data are merged for reporting of whole chromosome and/or segmental imbalances. (B) Information on whether the minimal size of segmental imbalances that can be detected is included in the report. This question specifically addressed those centres that report segmental imbalances. (C) Specification of whether the degree of mosaicism is reported. This question specifically addressed those centres that report mosaicism. (D) Inclusion of a recommendation and/or prioritization for embryo transfer in the genetic report. Question was multiple choice, with the different answer combinations represented in the figure. (E) Results on the question (yes/no) of whether mosaic embryos are considered in making a recommendation for embryo transfer, with indication of the most relevant comments. All data are presented as numbers and percentages. PGT, preimplantation genetic testing; PGT-A, PGT for aneuploidy; PGT-M, PGT for monogenic/single gene defects; PGT-SR, PGT for chromosomal structural rearrangements.
Embryo transfer strategy
Of 187 respondents, 119 (63.6%) indicated that they do not have a written embryo transfer strategy for all indications, or the strategy does not include management of mosaic embryos. However, the importance of genetic counselling was highlighted throughout. In some cases, the available written strategy specifies either transfer of mosaic only if no euploid embryo(s) are available, or transfer of euploid embryo(s) only, with no transfer of mosaics.
The preferred embryo transfer strategy for all indication types is vitrification of embryos immediately after biopsy and warming/transfer in a later frozen embryo transfer cycle (∼ 90% of centres). The majority of centres apply a single embryo transfer strategy (87.7%) (Supplementary Data SV).
Transfer of mosaic embryos
When questioned on their experience with the transfer of mosaic embryos, 52.9% (102/193) of respondents stated that they had transferred a putative mosaic embryo and would do it again, while 9.8% (19/193) of respondents would never consider it (Supplementary Data SVI).
In case of cycles where no euploid embryos and only mosaic embryos are available for transfer, the preferred option is embryo transfer/storing of a single embryo with ranking. Other options for the mosaic embryo are discard/donation to research or rebiopsy.
When both euploid and mosaic embryos are available for transfer, the preferred option is still to transfer/store a single embryo with ranking. From the survey replies, it is unclear whether this decision is taken in consultation with the patient or whether this is a decision solely taken by the laboratory. Half of the centres store at least one mosaic embryo, but an extra 30% consider that storing a second mosaic embryo with ranking is also an option (Fig. 4).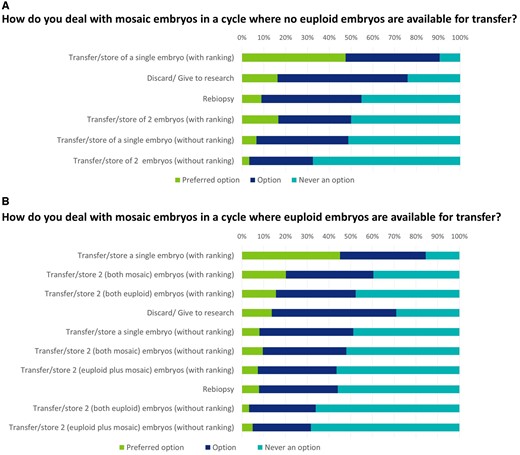
Patient counselling and informed consent
Patient counselling occurs at one timepoint, i.e. before the start of the PGT cycle (46.7%; 77/165) or at two timepoints, before the start of the PGT cycle and before embryo transfer (44.2%; 73/165) and often includes discussion of the fate of mosaic embryos. The transfer of mosaic embryos is often covered in the general informed consent (52.7%; 87/165), but sometimes a separate/additional informed consent is used (33.9%; 56/165) (Fig. 5).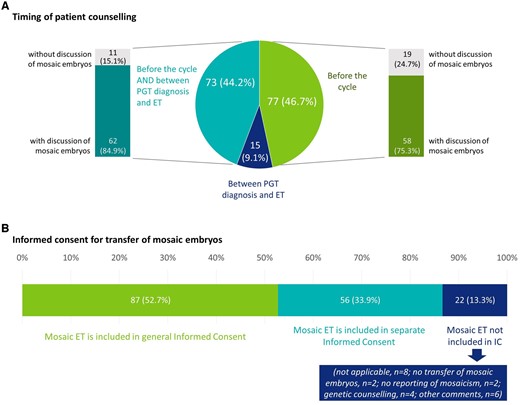
Figure 5.
Current practice with regards to patient counselling and informed consent. (A) Data on the timing of patient counselling, and whether it is performed before the cycle, between PGT diagnosis and ET, or at both timepoints. For each timepoint, the results were linked with the question whether or not mosaicism was discussed in counselling. (B) Data on whether IC on transfer of mosaic is separate, or included in the general informed consent (single choice question). All data are represented as numbers and percentages. IC, informed consent; ET, embryo transfer; PGT, preimplantation genetic testing.
Prenatal testing and children follow-up
Following the transfer of a mosaic embryo, prenatal diagnosis was recommended in 95% (151/159) of centres. In 62.9% (100/159) of all centres, amniocentesis (alone or in combination with other tests) is recommended, while 32.1% (51/159) of centres recommend non-invasive prenatal test (NIPT) and/or chorionic villus sampling (CVS) (but no amniocentesis) (Supplementary Data SVII). When requesting more details through an open question, 106 replies were received of which 87.7% (93/106) provided a strategy for prenatal diagnosis and 12.3% (13/106) specified either that they do not provide any recommendation or that the recommendation depends on the treating gynaecologist. Of those centres providing a strategy, the 38.7% (36/93) indicated amniocentesis and 32.2% (30/93) indicated NIPT as the strongest recommendation. NIPT was most often (n = 28) recommended on its own but also with a recommendation of further testing if an abnormality is detected (n = 2). Nine centres (9.7%; 9/93) replied that they recommended NIPT in combination with CVS or amniocentesis, regardless of NIPT findings.
Live births following transfer of mosaic embryos were reported in 64.2% (68/106) of the centres. Without further information on how many mosaic embryos have been transferred and how many of these resulted in a live birth, a live birth rate (LBR) following mosaic embryo transfer could not be calculated.
Follow-up of pregnancies/children after transfer of a mosaic embryo is performed in many centres (68.9%; 73/106), although 19.8% (21/106) of centres indicate that information on many children is lost during follow-up (Supplementary Data SVII).
Current data on chromosomal mosaicism
From the literature search, 7623 references were retrieved of which 6085 were excluded based on predefined criteria. An additional 1306 references did not focus on mosaicism and PGT. For the remaining 232 papers, full texts were retrieved, and the most significant papers are summarized below.
Outcomes of ‘mosaic’ embryo transfer following TE biopsy
A prospective multicentre study and combined meta-analysis reported consistently lower LBRs and higher miscarriage rates with mosaic embryo transfer, but also commented that transfer of mosaic embryos could still be an option for couples with no euploid embryos after PGT-A (Zhang et al., 2020). Another large retrospective study reported statistically significant lower ongoing pregnancy rate (OPR)/LBRs following transfer of mosaic embryos as compared to euploid embryos (37.0% versus 52.3%), with higher miscarriage rates (20.4% versus 8.6%) (Viotti et al., 2021b). Similar results were reported from a subanalysis of 164 mosaic embryos used without apparent selection bias, i.e. those used at the first transfer (Viotti et al., 2021b). Most recently, a prospective and double-blinded non-selection trial found that putative mosaic embryos in the low (20–30%) to moderate-range (30–50%) resulted in LBRs similar to euploid ones, and showed no increase in miscarriage risk and no cases of mosaicism or UPD in the subset of newborns for which follow-up testing could be performed (Capalbo et al., 2021).
On the safety side, a mosaic PGT-A result (based on an intermediate copy number value in the clinical TE biopsy) has a very low predictive value with respect to the detection of true mosaicism in the corresponding foetus or newborn. There is currently no evidence suggesting that offspring from low-range mosaic embryo transfer are at greater risk for this outcome compared to those conceived from euploid/untested embryos. There are only two published case reports describing the confirmation of the TE mosaic findings at a later stage of the pregnancy and postnatally (Kahraman et al., 2020) or postnatally only (Schlade-Bartusiak et al., 2022). In contrast, studies have reported that the transfer of embryos with a purely euploid TE biopsy result does not prevent mosaicism in the forthcoming pregnancy (Friedenthal et al., 2020; Capalbo et al., 2021).
There are relatively few data on the outcomes after transfer of putative mosaic embryos with regards to the health of pregnancies and children, but available data seem to be reassuring. Putative mosaic embryos seem either to lead to implantation failure/pregnancy loss, or to result in a live birth with no apparent abnormality.
Clinical validity of detecting mosaicism at TE biopsy
Studies have suggested that high-range mosaicism may represent a technical variation from the uniform aneuploidy range (Capalbo et al., 2021; Handyside et al., 2021; Wu et al., 2021), but also that TE biopsy—inner cell mass (ICM) concordance rates are poor for TE biopsies showing mosaicism, and decrease with the use of more dynamic ranges for mosaicism classification, such as reporting mosaicism from 20% to 80% (Chuang et al., 2018; Popovic et al., 2018; Fragouli et al., 2019; Navratil et al., 2020). Only a few studies have evaluated the concordance of a mosaic TE result with the rest of the embryo and with the ICM, but they are illustrative for this poorer performance. The studies with quantified results are summarized in Table II. A study on embryonic outgrowth on Day 12 has consistently found poor positive predictive value (PPV) for putative mosaic findings between TE biopsy and the outgrowth (Popovic et al., 2019).
Table II
Concordance of mosaic results at TE biopsy with the rest of the embryo/ICM.
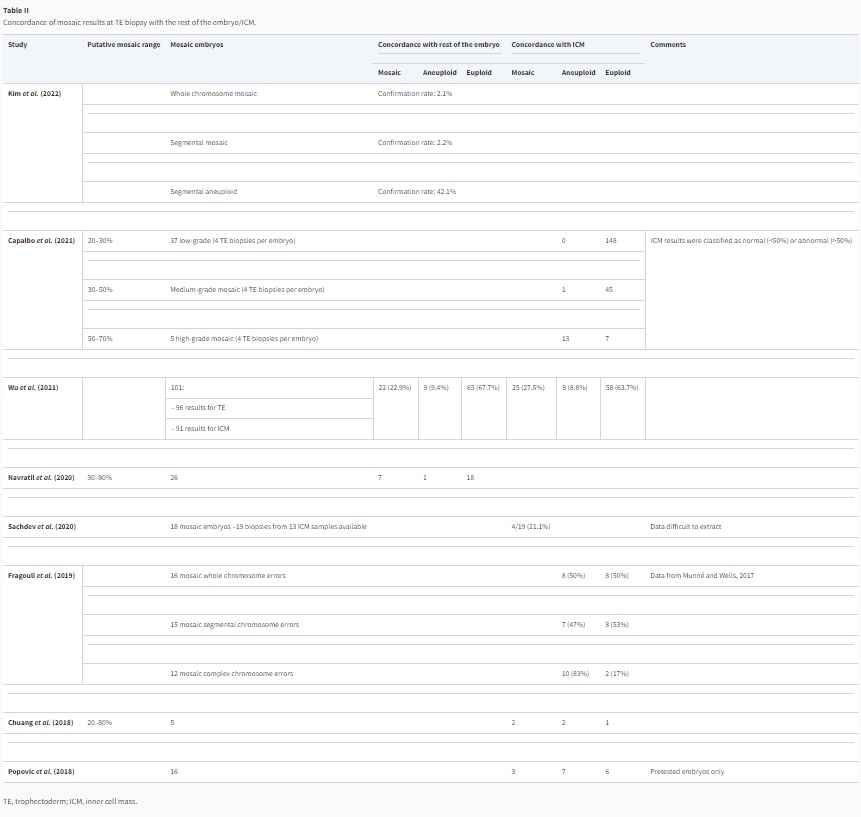
( Open in new tab )
Another aspect in the accuracy of detecting mosaicism at TE biopsy is the reproducibility, which can be assessed through performing rebiopsy of embryos designated as mosaic. In a recent review, the retest concordance analysis was performed for euploid, aneuploid and mosaic TE biopsy results (Marin et al., 2021). Based on 26 included studies, the review included rebiopsy data for 289 mosaic embryos, of which 123 were mosaic at retest, 84 euploid and 82 full aneuploid, resulting in a concordance rate of 42.6%. For euploidy and full aneuploidy, concordance rates were 93.7 and 81.4%, respectively. The study of Wu et al. additionally reported that the concordance rate of segmental chromosome mosaicism was significantly lower than whole chromosome mosaicism, and that the concordance rate was associated with the level of mosaicism (Wu et al., 2021).
Data on a cut-off for designating mosaicism
Studies have mostly evaluated cut-offs of 40% and 50% to define low-range and high-range mosaicism. A cut-off of 40% divides the ‘putative mosaic embryos’ into low-range mosaic (abnormal cells between 20 and 39%) and high-range mosaic embryos (abnormal cells between 40% and 80%). A recent meta-analysis evaluated the relevance of such a cut-off in predicting the outcomes (OPR, LBR and miscarriage rate) (Mourad et al., 2021). When a 40% cut-off value was used, the meta-analysis showed no difference in the outcomes after low- or high-range mosaic embryo transfer. When a cut-off of 50% was employed, the low-range mosaic embryos showed a higher OPR, and lower miscarriage rate, at least for the studies using the NGS technique. A similar observation, supporting a cut-off of 50%, was made in the retrospective study of Viotti et al. (2021b).
A recent non-selection trial reported no difference between the outcomes (LBR, OPR, clinical pregnancy rate, multiple pregnancy rate, miscarriage rate) after transfer of a low-range mosaic embryo (20–30% abnormal cells) and a moderate-range mosaic embryo (30–50%) (Capalbo et al., 2021).
A rebiopsy study showed that increasing levels of mosaicism were linked to increasing rates of full aneuploidy; for a mosaic level ≥60%, the full aneuploidy rate was ≥37.5% (Wu et al., 2021).
Currently, experience on higher range mosaic embryo transfers is limited (Leigh et al., 2022), but studies have suggested that high-range mosaicism detection (c. >50% aneuploid cells) in the original TE biopsy is associated with whole chromosome aneuploidy in a significant proportion of cases (Capalbo et al., 2021; Handyside et al., 2021; Wu et al., 2021), suggesting that high-range mosaic TE biopsies might actually represent technical variation from the uniform aneuploidy range.
Other aspects of chromosomal mosaicism were not extensively covered in the literature (e.g. association with embryo quality and prenatal diagnostic findings), and hence were explored and described as recommendations for future research.
Discussion
Chromosomal mosaicism is a secondary phenomenon to PGT, and therefore always linked to genetic testing. The discussion of the application, clinical benefit and utility of PGT (and specifically of PGT-A) is outside the scope of the current article. The article is restricted to providing guidance on the management of embryos with a mosaic test result after TE biopsy and PGT, without making any reference to the reasons or indications for the genetic test.
Still, the PGT modality is important when considering mosaicism. In clinical practice, PGT-A is widely performed with the goal of detecting aneuploidies in embryos and withholding them from transfer under the hypothesis that embryos with a chromosomally abnormal test result would lead to implantation failure, miscarriage or an ongoing aneuploid pregnancy/birth. As PGT-A is the most commonly performed PGT modality (Patrizio et al., 2019; Theobald et al., 2020; van Montfoort et al., 2021), there is an imbalance in the published data available with regards to chromosomal mosaicism, which may lead to the incorrect perception that it is a phenomenon unique to PGT-A, while it is also detected in PGT-M/PGT-A and PGT-SR; with regards to the latter PGT modalities, the technologies used and the goals are not the same. Patients opting for PGT-M have a genetic problem (high risk of transmitting a gene defect to the next generation) but not necessarily an infertility problem. Similarly, PGT-SR is performed upon indication, for example in patients with a balanced translocation, and aims to withhold from transfer those embryos that reveal unbalanced translocations leading to miscarriage, stillbirth or severe disabilities of children born.
For general discussion, we have summarized published data on the accuracy, analytical validity, clinical validity and clinical utility of chromosomal mosaicism detection. Both biological factors and technical artefacts may significantly limit the accuracy with which such chromosomal mosaicism and segmental imbalances can be detected (ESHRE PGT-SR/PGT-A Working Group et al., 2020).
With regards to the analytical validity of using intermediate copy number to determine the presence of mosaic chromosome abnormalities in TE biopsy specimens, technical/experimental noise, such as aneuploid TE biopsies with DNA contamination, as well as biological factors, such as polyploid embryos with extra/missing chromosomes, have been documented to complicate the designation of a mosaic result. Still, performing recommended validation experiments using mixtures of different ratios of normal and aneuploid cells to mimic mosaicism seems to provide an acceptable evaluation of technical accuracy (ESHRE PGT-SR/PGT-A Working Group et al., 2020; Viotti et al., 2021a). In a clinical setting, variability arises owing to the characteristics of TE biopsies of different cellularity/quality, changing reagent batches and shipping conditions.
With regards to clinical validity, one should consider the extent to which the TE biopsy accurately reflects the genetic status of the remainder of the embryo, especially the ICM, but also factors and technical limitations that may impact the analysis (technical artefacts, the biopsy technique, sample quality or contamination), and the validity of designating mosaicism and its range (Gleicher et al., 2017). It is clear that more studies are needed to determine concordance rates between TE with a mosaic result and ICM to confirm any clinical validity of detecting mosaicism.
Clinical utility can be defined as the likelihood of improved decision-making and enhanced clinical outcomes from considering mosaicism as a diagnostic criterion as compared to, or combined with, previous embryo selection criteria. With regards to the impact of transferring mosaic embryos on pregnancy outcomes (LBR, miscarriage rate), conclusions are difficult to draw as studies have used different inclusion criteria for patients, and different thresholds for designating mosaicism. It should also be considered that stringent measures for determining mosaicism and handling mosaic embryos may result in potential wastage of perfectly viable and healthy embryos and therefore have limited clinical utility and impact.
Some researchers have attempted to create embryo prioritization strategies based upon published data on outcome of mosaic embryo transfers (LBR, OPR, miscarriage rate), for example, prioritizing lower range mosaicism embryos over higher-range (with a cut-off of 50%), prioritizing segmental mosaics over whole chromosome mosaics, and single and double mosaics over complex mosaics (Mourad et al., 2021). However, given the paucity of well designed, prospective studies assessing the potential of mosaic embryos, it is unclear whether such strategies have any validity.
It is helpful to reiterate that chromosomal mosaicism can be detected for all three PGT modalities (PGT-A, PGT-SR and PGT-M). PGT centres should develop their own policy towards designating and reporting of mosaicism taking into consideration not only the PGT modality but also other factors, such as the biopsy method and procedure, results of validation experiments and the genetic testing platform, including its specific resolution and detection limits. In doing so, the multidisciplinary context of PGT and the patient’s needs are to be considered as well.
Recommendations
Recommendations for good practice in detecting, reporting and handling embryos with a TE result indicating chromosomal mosaicism have been formulated taking into consideration published data, data on practice derived from the survey, currently available good practice documents on PGT (ESHRE PGT Consortium Steering Committee et al., 2020; ESHRE PGT Consortium and SIG-Embryology Biopsy Working Group et al., 2020; ESHRE PGT-M Working Group et al., 2020; ESHRE PGT-SR/PGT-A Working Group et al., 2020) and/or accreditation requirements. Knowledge gaps are highlighted with accompanying recommendations for future research.
Owing to the variation in PGT modalities, biopsy methods and technologies, it is not possible to provide a standardized decision approach for managing chromosomal mosaicism. The recommendations formulated below aim to provide guidance to and be a basis for the centres’ own policy with regards to the management of ‘mosaic’ embryos. Upon considering the recommendation to develop the centre’s policy, one should ensure compliance with statutory requirements and/or clinical practice guidelines in the respective countries (Table III).
Table III
Recommendations for managing mosaicism in a PGT workflow.
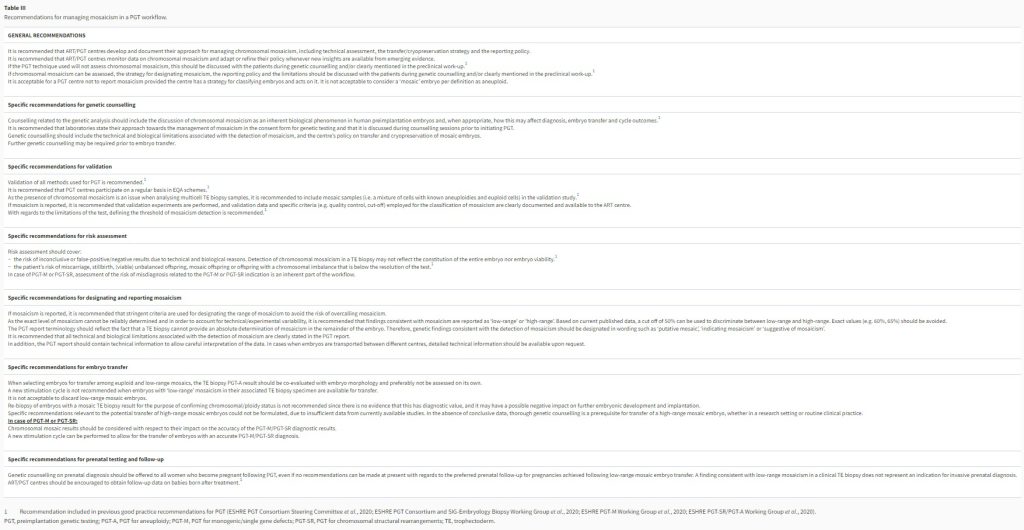
PGT, preimplantation genetic testing; PGT-A, PGT for aneuploidy; PGT-M, PGT for monogenic/single gene defects; PGT-SR, PGT for chromosomal structural rearrangements; TE, trophectoderm.
( Open in new tab )
Recommendations for future research
While shaping the recommendations, it became clear that many knowledge gaps remain and more research is warranted to leverage evidence-based medicine and decision-making. Here, we identify those gaps and provide directions on the way forward.
Questions remain with regards to the accuracy of mosaicism detection and the detection of mosaic segmental imbalances
Mosaicism detection is based on validation studies with cell mixtures, and while these provide a reasonably good model, they do not reflect the full extent and range of variation in biopsies containing only a limited number of cells. Larger datasets and application of different methodologies will help to resolve this uncertainty. Moreover, the accuracy of detection of specific subtypes of mosaicism (e.g. mosaic segmental imbalances) needs to be improved to be able to further assess the clinical validity. Such studies would complement validation studies measuring whole chromosome aneuploidies.
What is the value of novel genome amplification methods to improve mosaicism detection?
All clinically implemented PGT-A methods require genome amplification to provide sufficient DNA for the analysis. Current clinically used amplification methods are characterized by amplification artefacts, resulting in technical noise hampering interpretation and accurate detection of mosaicism as well as segmental (mosaic) imbalances (see also ESHRE PGT-SR/PGT-A Working Group et al., 2020). Novel methods that can generate genomic analysis without preamplification, which can address some of those issues, are being developed and pioneered (Zahn et al., 2017), but the potential for PGT remains to be determined.
What is the relevance of mosaicism with regards to embryo ranking and embryo transfer in PGT-A cycles?
There is evidence that low- to moderate-range mosaicism may not negatively affect LBRs. However, the consequences of high-range mosaicism (>50%) remain less well studied, but it can be hypothesized that the majority of high-range mosaic embryo(s) would be aneuploid and result in non-implantation or miscarriage. Still, high-range mosaic embryos could result in vital pregnancy and a healthy baby, and could therefore provide chances of a pregnancy in couples with no other options. With the modest PPV of low-range mosaicism, it is currently recommended to co-evaluate mosaicism with morphology. Aneuploid cells have a tendency to grow more slowly and, as a consequence of the different genetic constitution, they could have different cellular shapes (Martín et al., 2021; Zhang et al., 2022). However, much is still unknown on the link between morphology and genetic constitution, as well on the morphokinetic features. Further studies exploring the morphology of mosaic and non-mosaic embryos at different stages of development could provide some further clarity on the link between them, the relevance of mosaicism and morphology, and their interdependence.
What is the outcome with regards to pregnancy and offspring of embryo transfer with mosaic aneuploidies detected during PGT?
The level of mosaicism in TE biopsy has previously been related to the chance of live birth following embryo transfer, but still more studies would be relevant to confirm that the conclusions can be generalized to other settings (Spinella et al., 2018). Preferably, the topic should be investigated through prospective blinded non-selection trials that include low-range and/or high-range mosaic aneuploid embryos. Most of the currently available studies have focused on implantation and pregnancy, but not live birth and the health of the newborn. Another aspect to be included in research is the genetic analysis of pregnancy tissue (i.e. the product of conception) after pregnancy loss. Real-world data could provide more information on the pre- and postnatal outcomes of mosaic embryo transfer. Prenatal follow-up is largely implemented in clinical practice, but these data are not systematically recorded or shared with the scientific community. Only through the large-scale systematic registration of the degree and type of mosaicism in the transferred embryo, prenatal follow-up data and birth outcomes, will the full clinical consequences of mosaic embryo transfer be revealed. In such large-scale systematic registration, embryos diagnosed with (mosaic) segmental imbalances should also be included to clarify the fate of these embryos and could guide future decision-making with regards to their transfer.
To what extent does the TE biopsy reflect accurately the genetic status of the remainder of the embryo?
It is currently unknown to what extent the aneuploidy in a TE biopsy reflects the status of the ICM or the remainder of the TE. More studies are needed to investigate the concordance/discordance of the mosaicism status between TE/ICM as well as the fate of mosaic cells during pregnancy, while concurrently paying attention to potential differences between chromosomes. Data from model systems have indicated that aneuploid cells in TE and ICM may have a different fate and persistence during embryogenesis (Bolton et al., 2016; Yang et al., 2021), and that different chromosomal aneuploidies may have distinct developmental potential among different tissues (Shahbazi et al., 2020).
Would the use of comprehensive PGT, currently applied for PGT-M, influence the current best practice recommendations and observations with regards to mosaicism?
The current PGT-A standard of care relies on low-pass sequencing methods to determine the ploidy status of the different chromosomes. For PGT-M purposes, laboratories are gradually opting for techniques that combine genome-wide haplotyping and aneuploidy detection (i.e. comprehensive PGT) (Handyside et al., 2010; Vermeesch et al., 2016). As a consequence, aneuploidies can be classified as being meiotic or mitotic in origin (Vermeesch et al., 2016). Since a meiotic error is passed from the gametes to the embryo, it is more likely to be present in a majority of cells as compared to a mitotic error. As a consequence, the viability of mosaic meiotic aneuploidy might be different as compared to mosaic mitotic aneuploidy. With growing number of comprehensive PGT being performed on blastocysts biopsies and equally a growing number of mosaic aneuploidy embryos transferred, these data may become available (Verdyck et al., 2022). Similarly, it might be valuable to start large-scale clinical trials to evaluate the added value of haplotype-based mosaicism detection.
What is the value of NIPT in the prenatal follow-up after mosaic embryo transfer?
Standard NIPT testing and CVS are probably not the best options for prenatal testing after transfer of a mosaic embryo (Spinella et al., 2018; Victor et al., 2019). Genome-wide NIPT (24 chromosome NIPT methodology) allows accurate detection of placental mosaicism for rare autosomal trisomies (Brison et al., 2018). Genome-wide NIPT may therefore be a first monitoring system to identify potentially affected foetuses. Broadscale analyses might provide unbiased views of the viability of different types of mosaicism.
What is the value of reporting low-range mosaicism?
Genetic findings should not be reported simply because they are observed but because they have demonstrable clinical utility. On the other hand, there is also the duty to report clinically and biologically relevant results to patients. The tension between providing information and limited clinical relevance raises question on the benefit for both the couples and the professionals, which can be explored in qualitative studies and further corroborated in ethical discussion and professional guidance.
Supplementary data
Supplementary data are available at Human Reproduction Open online
Data Availability
The data underlying this article are available in the article and in its online supplementary material.
Acknowledgements
The authors would like to thank the 239 respondents who completed the survey for their time and effort, and the 16 experts who contributed to the stakeholder review for their helpful comments and suggestions; the list of these reviewers is available in Supplementary Data SVIII.
Authors’ roles
M.D.R. and G.K. were responsible for the design of the survey and the paper and for managing the working group. N.V. and S.M. provided methodological support. All other authors contributed equally in writing the paper, and all approved the final version for publication.
Funding
The meetings and technical support for this project were funded by the European Society of Human Reproduction and Embryology.
Conflict of interest
M.D.R. participated in the EQA special advisory group, outside the submitted work and is the chair of the PGT working group of the Belgian society for human genetics. D.W. declared receiving salary from Juno Genetics, UK. A.C. is an employee of Igenomix, Italy and C.R. is an employee of Igenomix, Spain. C.S. received a research grant from FWO, Belgium, not related to the submitted work. I.S. declared being a Co-founder of IVFvision Ltd, UK. J.R.V. declared patents related to ‘Methods for haplotyping single-cells’ and ‘Haplotyping and copy number typing using polymorphic variant allelic frequencies’, and being a board member of Preimplantation Genetic Diagnosis International Society (PGDIS) and International Society for Prenatal Diagnosis (ISPD). K.S. reported being Chair-elect of ESHRE. The other authors had nothing to disclose.
Footnotes
†
ESHRE pages content is not externally peer reviewed. The manuscript has been approved by the Executive Committee of ESHRE.
References
_______________________________________________________________________
Google Scholar Crossref PubMed WorldCat
Google Scholar Crossref PubMed WorldCat
Google Scholar Crossref PubMed WorldCat
Google Scholar Crossref PubMed WorldCat
Google Scholar Crossref PubMed WorldCat
Google Scholar Crossref WorldCat
Google Scholar Crossref WorldCat
Google Scholar Crossref WorldCat
Google Scholar Crossref PubMed WorldCat
Google Scholar Crossref PubMed WorldCat
Google Scholar Crossref PubMed WorldCat
Google Scholar Crossref PubMed WorldCat
Google Scholar Crossref PubMed WorldCat
Google Scholar Crossref PubMed WorldCat
Google Scholar Crossref PubMed WorldCat
Google Scholar Crossref WorldCat
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
